Group Picture

April 2025
Our Research
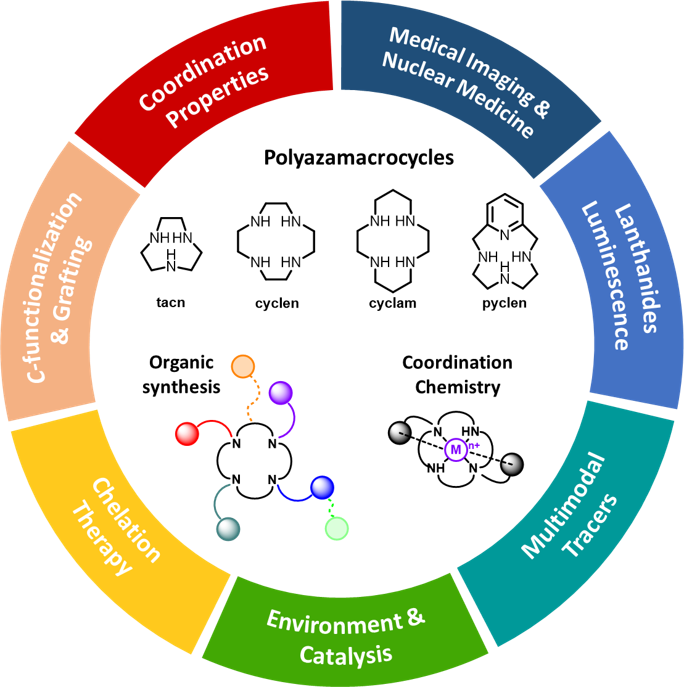
We are interested in the chemistry of polyazamacrocycles, i.e. cyclic compounds containing at least 9 atoms and 3 donors, that have exceptional properties for the chelation of a wide range of cations (transition and post-transition metals, lanthanides). Our core expertise lies in the domains of :
- Organic & macrocyclic synthesis : development of synthetic tools for the regioselective N-functionalization of the macrocyclic platforms, C-functionalization & aryl-functionalization strategies for the preparation of graftable/bifunctional chelators.
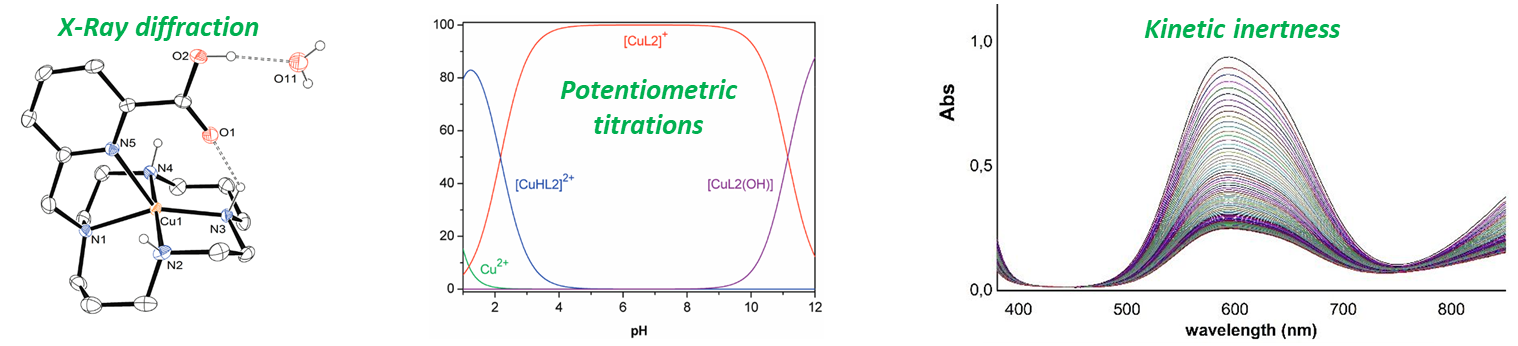
- Coordination chemistry : investigation of the chelators properties towards various cations, such as thermodynamic association constants (by potentiometry, UV-Vis & NMR spectroscopies), kinetic inertness in competitive media or structural studies (X-Ray diffraction, EPR spectroscopy).
With these tools, we specifically design chelators containing coordinating units to accommodate a designated cation, and additional chemical functions necessary for the desired application in various domains such as medicine, materials, catalysis or environment-directed processes.
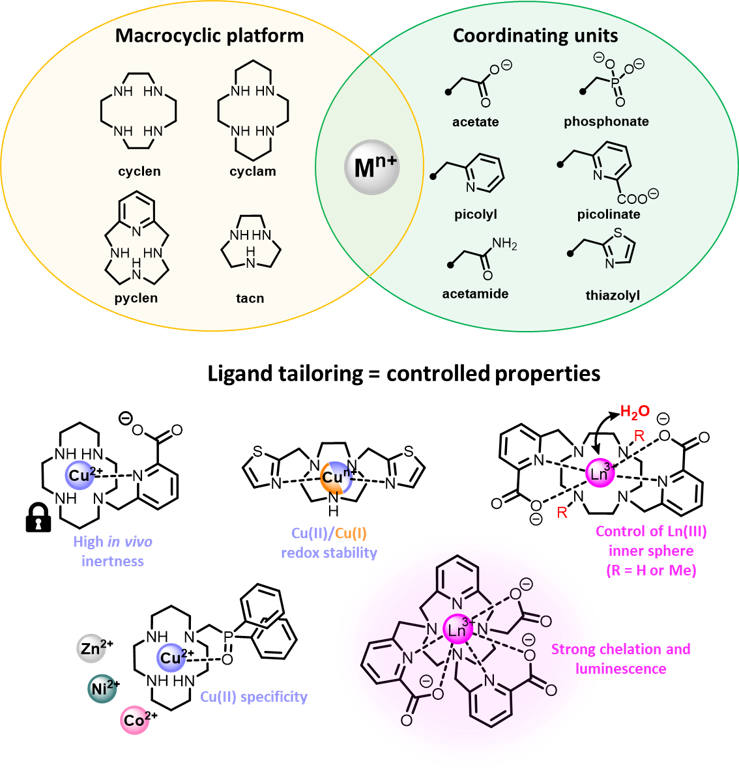
Thanks to a large variety of organic/macrocyclic synthetic tools, we are able to regioselectively functionalize the macrocyclic platforms with selected sets of coordinating "arms" (e.g. acetate, picolyl, picolinate...) that allow us to finely tune the coordination properties of the chelators. Playing on the denticity and the hard/soft donor character of these ligands thus leads to a fine control of the chelate properties.
For instance, the use of bidentate picolinate units on the different platforms has lead to highly robust chelators for a wide range of cations, such as copper(II) (Inorg. Chem. 2012) or lanthanides(III) (Inorg. Chem. 2018; Inorg. Chem. 2020). Subtle modification of the chelator substitution pattern can also allow precise control of the inner coordination sphere of the lanthanide cations, with coordination or absence of a water molecule (Inorg. Chem. 2012), a feature that has a key impact in applications such as MRI (Magnetic Resonance Imaging) or luminescence.
The use of less common coordinating arms can also trigger peculiar properties to the chelates, as we have demonstrated for example with the use of thiazolyl groups that provide remarkable Cu(II)/Cu(I) redox stability (Dalton Trans. 2016), phosphine oxide pendants that allow specific coordination of Cu(II) ions versus their divalent congeners (Zn(II), Co(II), Ni(II)) (Inorg. Chem. 2023), or tridentate pyridylphosphonates that lead to the controlled formation of supramolecular constructs with lanthanide ions (Inorg. Chem. 2020; Inorg. Chem. 2023).
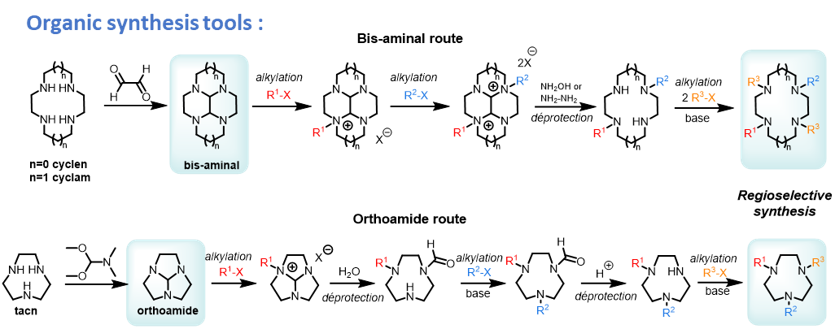
All these chelators are prepared thanks to a range of organic synthesis tools, that allow regioselective installation of the coordinating/functional groups.
Main collaborations : Pr. Carlo Platas-Iglesias (La Coruna, Spain), Pr. Gyula Tircso (Debrecen, Hungary), Pr. Rita Delgado (Lisbon, Portugal), Dr; Loïc Charbonnière (IPHC Strasbourg)
References :
- L. M. P. Lima, D. Esteban-Gomez, R. Delgado, C. Platas-Iglesias, R. Tripier, Inorg. Chem. 2012, 6916.
- M. Le Fur, E. Molnar, M. Beyler, O. Fougère, D. Esteban-Gomez, O. Rousseaux, R. Tripier, G. Tircso, C. Platas-Iglesias, Inorg. Chem. 2018, 6932.
- Z. Garda, V. Nagy, A. Rodriguez-Rodriguez, R. Pujales-Paradela, V. Patinec, G. Angelovski, E. Toth, F. K. Kalman, D. Esteban-Gomez, R. Tripier, C. Platas-Iglesias, G. Tircso, Inorg. Chem. 2020, 8184.
- A. Rodriguez-Rodriguez, D. Esteban-Gomez, A. de Blas, T. Rodriguez-Blas, M. Fekete, M. Botta, R. Tripier, C. Platas-Iglesias, Inorg. Chem. 2012, 2509.
- M. Le Fur, M. Beyler, N. Le Poul, L. M. P. Lima, Y. Le Mest, R. Delgado, C. Platas-Iglesias, V. Patinec, R. Tripier, Dalton Trans. 2016, 7406.
- M. M. Le Roy, S. Héry, N. Saffon-Merceron, C. Platas-Iglesias, T. Troadec, R. Tripier, Inorg. Chem. 2023, 8112.
- R. C. Knighton, L. K. Soro, T. Troadec, V. Mazan, A. M. Nonat, M. Elhabiri, N. Saffon-Merceron, S. Djenad, R. Tripier, L. J. Charbonnière, Inorg. Chem. 2020, 10311.
- N. Hamon, L. Godec, E. Jourdain, F. Lucio-Martinez, C. PLatas-Iglesias, M. Beyler, L. J. Charbonnière, R. Tripier, Inorg. Chem. 2023, 18940.
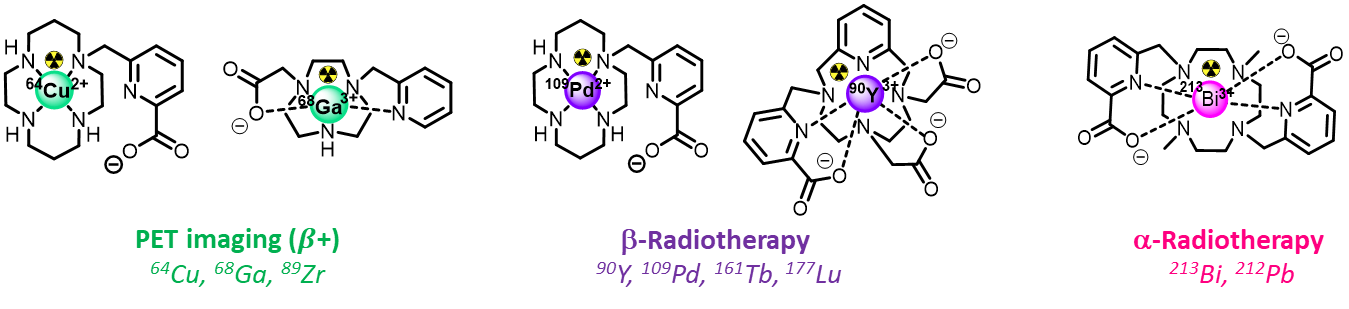
Selected references (Figure) : 64Cu : Nucl. Med. Biol. 2014 ; 68Ga : Inorg. Chem. 2023 ; 109Pd : Chem. Eur. J. 2022 ; 90Y : Chem. Commun. 2017 ; 213Bi : Chem. Commun. 2012
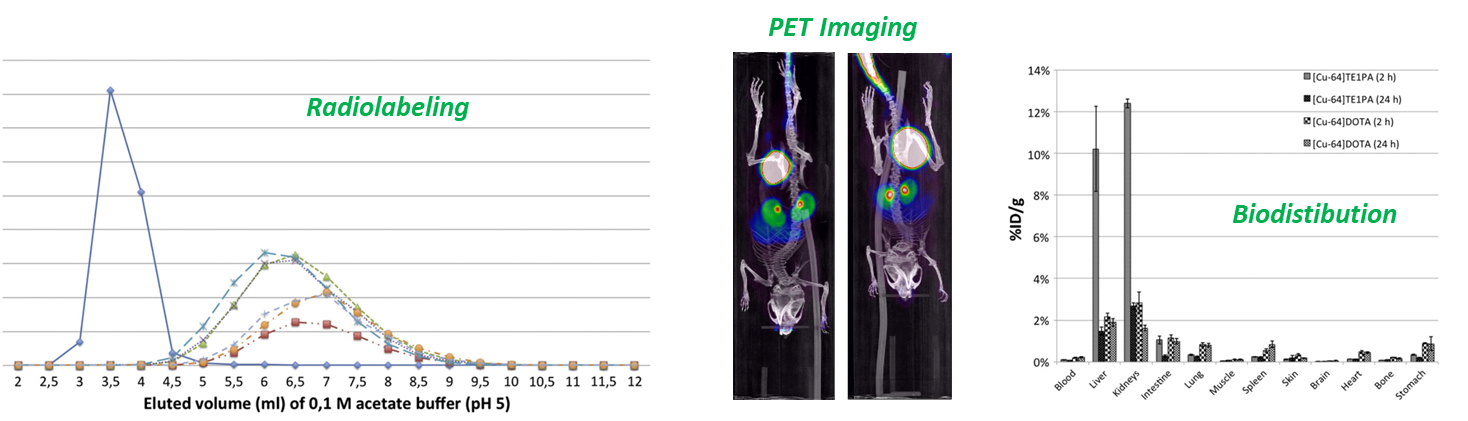
We apply our coordination chemistry expertise to the design of robust chelators for metallic cations of interest in Nuclear Medicine. We are particularly interested in three types of radionuclides : beta+ (positons) emitters that allow Positon Emission Tomography (PET Imaging), beta- and alpha emitters that are used in Internal Targeted Radiotherapy.
In this field, to avoid uncontrolled release of the cations in vivo, leading to non-specific action and potential toxicity, performant chelators are required to fulfill a set of characteristics, :
High thermodynamic stability
Fast Radiolabeling in mild conditions
High kinetic inertness in competitive media
Good clearance from the body
Therefore, we design chelators from the appropriate macrocyclic platforms and sets of coordinating arms to provide an optimized coordination sphere to the targeted cations in terms of geometry, coordination number, hard/soft character...
We first study the coordination properties, thermodynamic and kinetic parameters in-house on "cold" (i.e. non-radioactive) cations by multinuclear NMR, X-Ray diffraction analysis, UV-visible spectroscopy and potentiometric titrations.
Then the radiolabeling and biological experiments are conducted via our large collaborative network : Prs. A. Faivre-Chauvet & M. Chérel (CRCI2NA, Nantes), Dr. N. Lepareur (Centre E. Marquis, Rennes), Dr. M. Bartholomä (Homburg, Germany), Dr. E. Jestin (CyROI LA Réunion), NECSA (South Africa)...
References :
- M. Frindel, N. Camus, A. Rauscher, M. Bourgeois, C. Alliot, L. Barré, J6F. Gestin, R. Tripier, A. Faivre-Chauvet, Nucl. Med. Biol. 2014, e49.
- A. Marlin, A. Koller, E. Madarasi, M. Cordier, D. Esteban-Gomez, C. Platas-Iglesias, G. Tircso, E. Boros, V. Patinec, R. Tripier, Inorg. Chem. 2023, 20634.
- J. Pineau, L. M. P. Lima, C. Platas-Iglesias, J. R. Zeevaart, C. S. Driver, N. Le Bris, R. Tripier, Chem. Eur. J. 2022, e202200942.
- M. Le Fur, M. Beyler, E. Molnar, O. Fougère, D. Esteban-Gomez, G. Tircso, C. Platas-Iglesias, N. Lepareur, O. Rousseaux, R. Tripier, Chem. Commun. 2017, 9534.
- L. M. P. Lima, M. Beyler, F. Oukhatar, P. Le Saëc, A. Faivre-Chauvet, C. Platas-Iglesias, R. Delgado, R. Tripier, Chem. Commun. 2014, 12371.
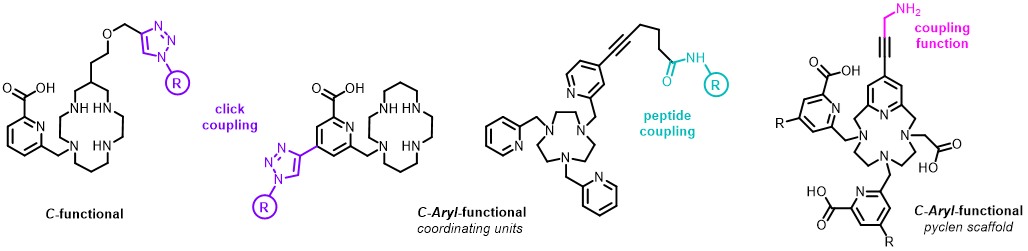
To use our chelators in vivo for targeted applications (Imaging or Therapy), bifunctional versions (including a coupling function) are required for grafting on targeting molecules of interest (antibodies, peptides, organic compounds). These specifically bind to biomarkers/receptors overexpressed by pathological cells (cancer cells in particular), allowing accurate diagnosis and personalization of the treatments.
First, we have demonstrated through our studies on the 64Cu-te1pa chelate, that coordination properties of the chelator was fully preserved via C-functionalization of the chelator, as opposed to N-functionalization or grafting through the picolinate coordinating group (J. Org. Chem. 2014; RSC Advances 2017; Org. Biomol. Chem. 2018).
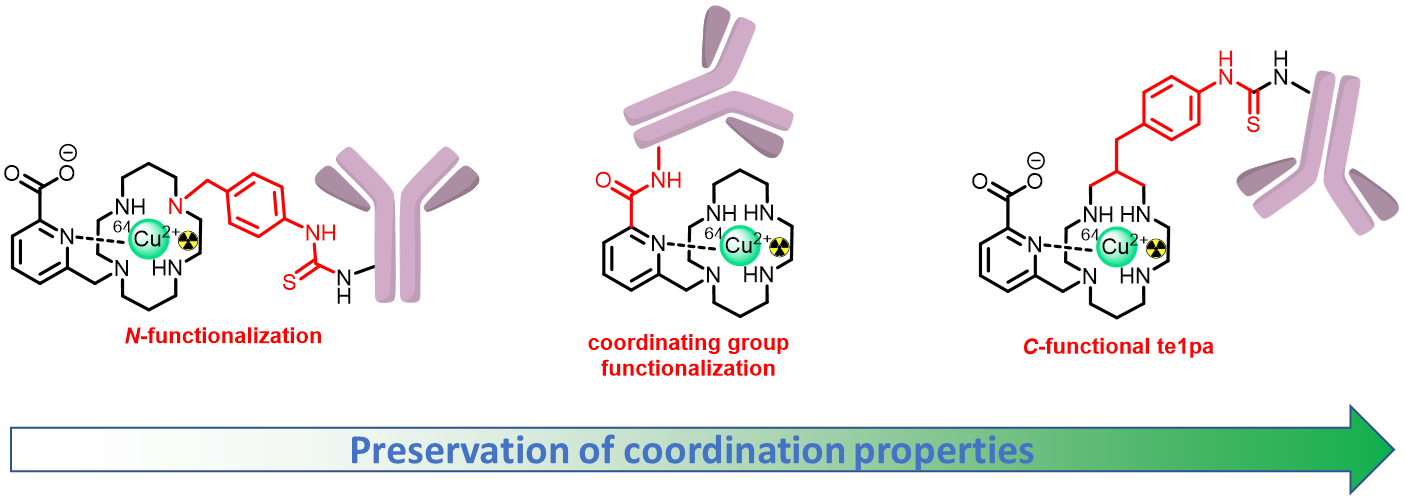
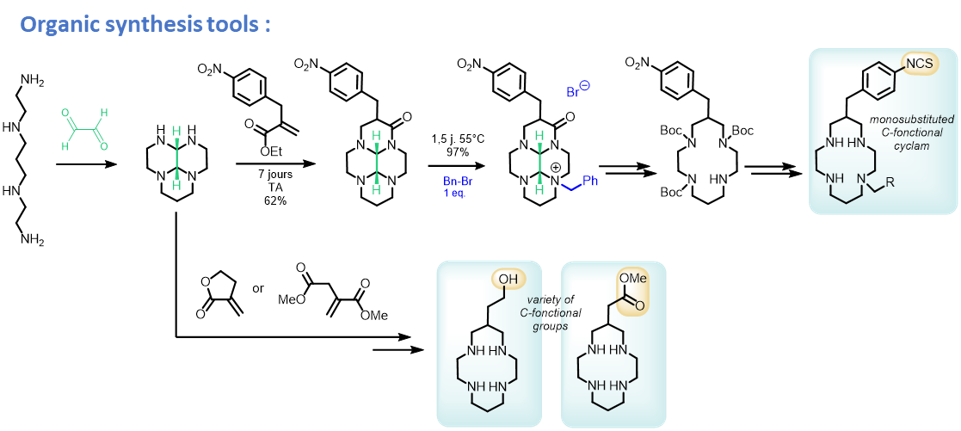
We have now enlarged the scope of this C-functionalization, with different coupling functions (Cu-AAC click, J. Org. Chem. 2024; Chem. Commun. 2023) and a synthesis-friendly variation via C-Aryl-functionalization on the pendant groups (Chem. Commun. 2023; Bioconjugate. Chem. 2022) or the pyclen scaffold (J. Org. Chem. 2023).
References :
- N. Camus, Z. Halime, N. Le Bris, H. Bernard, C. Platas-Iglesias, R. Tripier, J. Org. Chem., 2014, 1885.
- M. Frindel, P. L. Saëc, M. Beyler, A.-S. Navarro, C. Saï-Maurel, C. Alliot, M. Chérel, J.-F. Gestin, A. Faivre-Chauvet, R. Tripier, RSC Adv., 2017, 9272.
- T. Le Bihan, A.-S. Navarro, N. L. Bris, P. L. Saëc, S. Gouard, F. Haddad, J.-F. Gestin, M. Chérel, A. Faivre-Chauvet, R. Tripier, Org. Biomol. Chem., 2018, 4261.
- C. Ollier, A. Méndez-Ardoy, F. Ortega-Caballero, J. L. Jiménez-Blanco, N. Le Bris, R. Tripier, J. Org. Chem., 2024, 5988.
- J. Pineau, L. M. P. Lima, M. M. L. Roy, S. Marionneau-Lambot, M. Cordier, P. L. Saëc, J. R. Zeevaart, C. H. S. Driver, A. Faivre-Chauvet, N. L. Bris, R. Tripier, Chem. Commun., 2023, 888.
- A. Marlin, F. Le Pape, J. Le Goff, N. Hamon, T. Troadec, R. Tripier, C. Berthou, V. Patinec, Bioconjugate Chem., 2022, 1377.
- N. Hamon, L. Bridou, M. Roux, O. Maury, R. Tripier and M. Beyler, J. Org. Chem., 2023, 8286.
Optical Imaging :
Building on the exceptionnal coordination properties of picolinate-appended pyclen chelators (Inorg. Chem. 2018) for lanthanide(III) ions, we are currently developping highly luminescent probes for in vitro and in vivo bioimaging. As the direct excitation of lanthanides is a limiting factor for such applications, we are designing chelators bearing organic chromophores that play the role of antennae to harvest light and transfer energy to the lanthanide cation. In addition, we are here using 2-photon excitation, that allows better penetration in the tissues and a better spatial control of the excitation (J. Am. Chem. Soc. 2020).
Thanks to our expertise in bifunctional derivatives of chelators (see C-functionalization and grafting Section), we have also developed tools to graft these probes for targeted applications (J. Org. Chem. 2023).

The ligands synthesis and photophysical studies are conducted in close collaboration with the group of Dr. O. Maury (ENS Lyon).
Light Upconversion :
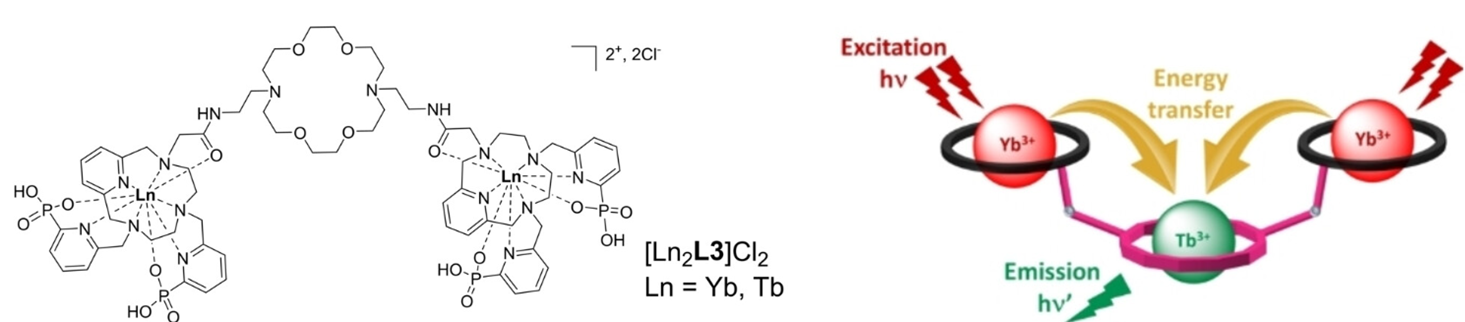
We are also exploring polynuclear lanthanide assemblies with various chelators (Inorg. Chem. 2020; Inorg. Chem. 2023), with a particular attention on light upconversion. This rare physical phenomenon relates to the absorption of two photons by a molecule, followed by emission of a single photon of higher energy/lower wavelength. This peculiar process has been observed for the first time in solution by the group of Drs. L. Charbonnière & A. Nonat (IPHC Strasbourg) through weak interactions between Yb(III) complexes and Tb(III) cations.
Through this collaboration, we have recently prepared various polytopic ligands that can sequentially coordinate different lanthanides and selectively lead to controlled Ln2Ln'L or Ln2Ln'2L assemblies, enhancing energy transfer processes between lanthanides in solution (Angew. Chem. Int. Ed. 2025).
References :
- M. Le Fur, E. Molnár, M. Beyler, O. Fougère, D. Esteban-Gómez, O. Rousseaux, R. Tripier, G. Tircsó and C. Platas-Iglesias, Inorg. Chem., 2018, 6932.
- N. Hamon, A. Roux, M. Beyler, J.-C. Mulatier, C. Andraud, C. Nguyen, M. Maynadier, N. Bettache, A. Duperray, A. Grichine, S. Brasselet, M. Gary-Bobo, O. Maury and R. Tripier, J. Am. Chem. Soc., 2020, 10184.
- N. Hamon, L. Bridou, M. Roux, O. Maury, R. Tripier and M. Beyler, J. Org. Chem., 2023, 8286.
- R. C. Knighton, L. K. Soro, T. Troadec, V. Mazan, A. M. Nonat, M. Elhabiri, N. Saffon-Merceron, S. Djenad, R. Tripier and L. J. Charbonnière, Inorg. Chem., 2020, 10311.
- N. Hamon, L. Godec, E. Jourdain, F. Lucio-Martínez, C. Platas-Iglesias, M. Beyler, L. J. Charbonnière and R. Tripier, Inorg. Chem., 2023, 18940.
- N. Hamon, L. Godec, S. Sanchez, M. Beyler, L. J. Charbonnière and R. Tripier, Angew. Chem. Int. Ed., 2025, e202414608.
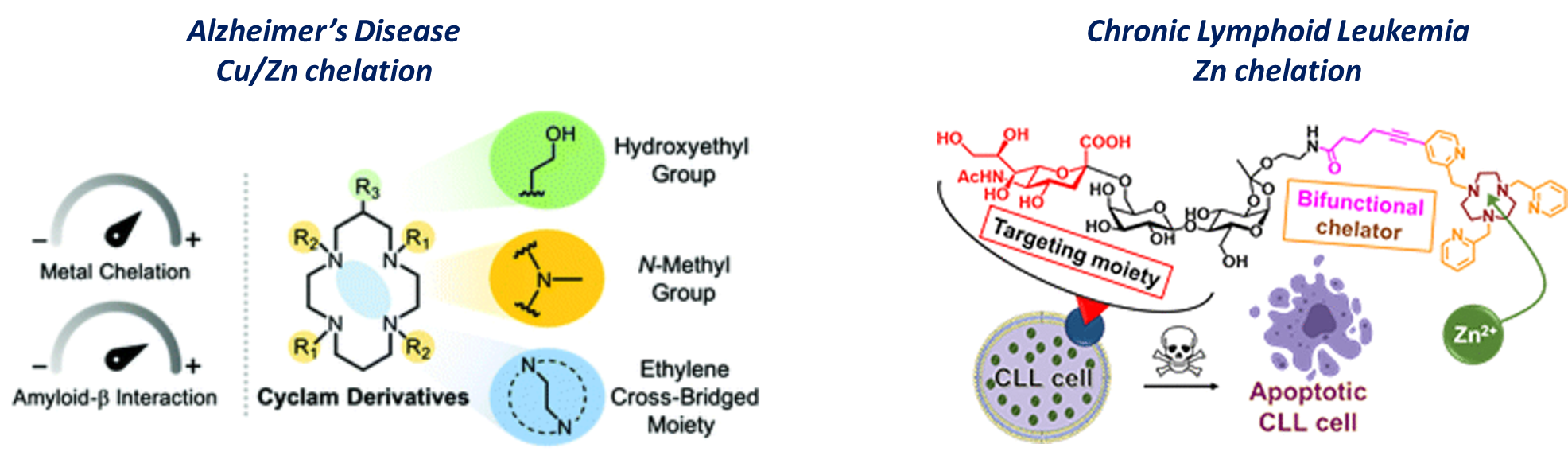
Most applications of chelators rely on the complexation of cations and radionuclides of interest, that are subsequently injected in vivo as chelates (i.e. complexed forms) to perform their action (Imaging, therapy...). However, some pathologies are strongly associated to misregulation of cations homeostasis that alter some physiological mecanisms in vivo. This is for instance the case in Alzheimer's Disease (AD), where zinc and copper cations play a key role in beta-amyloid aggregation that participates in the cognitive troubles of patients. In Chronic Lymphoid Leukemia (CLL), anormal intracellular zinc concentration has also been demonstrated to interfere in the apoptotic death of tumour cells, thus favouring their proliferation.
In both cases, chelation therapy, i.e. the use of chelators under their "free ligand" form to complex cations in vivo, is a very promising strategy.
However, the design of such chelators is challenging as they must fulfill a set of key parameters, such as selectivity for the targeted cations, solubility in biological media under their free form, and appropriate complexation kinetics.
Thus, we have been recently developping series of ligands for both applications, based on the cyclam scaffold for copper(II) and zinc(II) chelation in AD (Inorg. Chem. Front. 2020), and tacn scaffold for zinc(II) chelation in CLL (Bioconjugate Chem. 2022; Dalton Trans. 2025).
References :
- G. Kim, E. Lelong, J. Kang, J.-M. Suh, N. L. Bris, H. Bernard, D. Kim, R. Tripier and M. H. Lim, Inorg. Chem. Front., 2020, 4222.
- A. Marlin, F. Le Pape, J. Le Goff, N. Hamon, T. Troadec, R. Tripier, C. Berthou and V. Patinec, Bioconjug. Chem., 2022, 1377.
- A. Marlin, F. L. Pape, T. Troadec, J. Le Goff, R. Tripier, C. Berthou and V. Patinec, Dalton Trans., 2025, 3939.
A current challenge in the field of imaging and therapeutic probes is the design of multimodal tracers, combining different techniques with the same tracer biodistribution. These combinations can be of multiple imaging modalities (e.g. MRI/PET) or of an imaging modality and a therapeutic one, which is in this case called "theranostics" (e.g. PET/Radiotherapy, MRI/PDT...).
Therefore we have designing chelators for different combinations, for instance based on gadolinium(III) coordination (allowing Magnetic Resonance Imaging, MRI) and organic chromophores (allowing PhotoDynamic Therapy, PDT) for MRI/PDT theranostics (Inorg. Chem. Front. 2021),
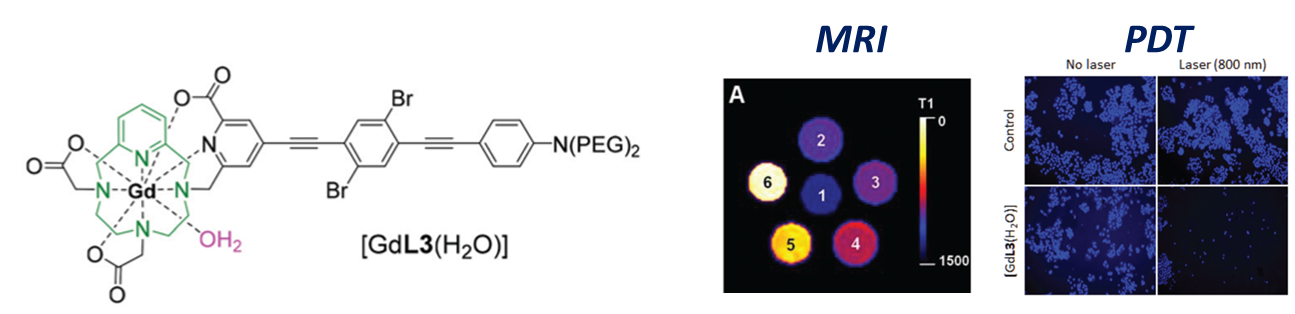
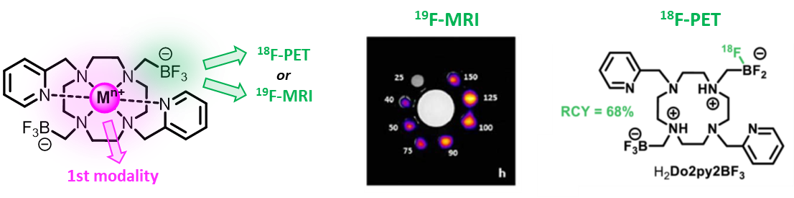
or by the introduction of trifluoroborate pendants on chelators, that will allow combinations of the cations properties with either 18F-PET or 19F-MRI (Chem. Sci. 2024).
References :
- L. Abad Galan, N. Hamon, C. Nguyen, E. Molnar, J. Kiss, J. Mendy, K. Hadj-Kaddour, M. Onofre, G. Trencsenyi, C. Monnereau, M. Beyler, G. Tircso, M. Gary-Bobo, O. Maury, R. Tripier, Inorg. Chem. Front., 2021, 2213.
- C. Sire, V. Meynerol, N. Saffon-Merceron, E. Terreno, F. Garello, L. Tei, E. Jestin, R. Tripier, T. Troadec, Chem. Sci., 2024, 13550.
CO2 reduction :
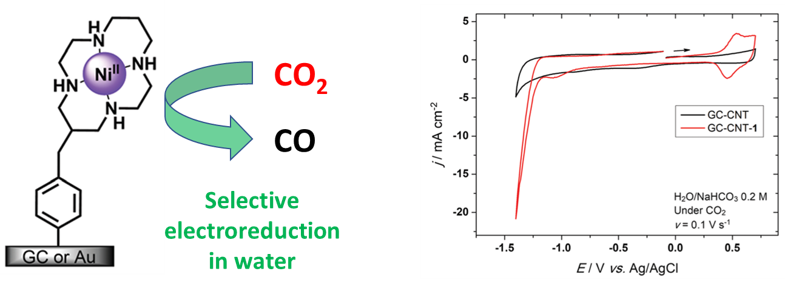
Nickel(II)-cyclam complex has been long-known as a CO2-electroreduction catalyst. However the selectivity of the reaction (formation of CO and other C1 products, H2 as byproduct) and the required overpotential are not favourable for a wide development. Owing to the opportunity offered by CO2-reduction and CO2-recycling as a potential mean to fight global warming, optimizing this reaction is a great challenge.
Nickel(II)-cyclam had already been immobilized on electrodes via N-functionalization of the chelators and grafting through non-covalent or reversible interactions, showing improvements on selectivity and reaction conditions. However we have recently achieved covalent linkage of the complex on a glassy carbon electrode, thanks to the C-functionalization tools developed in the group, that lead to the first heterogenized Ni complex that can perform selective reduction of CO2 to CO in water and in mild conditions (Chem. Commun. 2022).
This work is carried out in collaboration with the group of Dr. N. Le Poul from our research unit (UMR 6521 CEMCA) in Brest.
MRI contrast agents recycling :
Pr. R. Tripier has recently been awarded the joint Chair "MeGadoRe" (for Medical Gadolinium Recycling) alongside Pr. Douraied Ben Salem (UBO/CHU Brest) and Pr. J-A. Barrat (IUEM, UBO), which holds the ambitious goal of collecting and recycling gadolinium contrasts agents that are left over after patient injection, and excreted by patient after the imaging.

This chair is supported by Fondation UBO and private/industrial partners : Guerbet, Bracco, Imadis and Crédit Mutuel Arkéa.
website : megadore.org
References :
- A. Forget, M. Regnacq, C. Orain, E. Touzé, E. Lelong, C. Brandily, H. Bernard, R. Tripier, N. Le Poul, Chem. Commun., 2022, 6785.
Alumni :
PhDs (since 2010) :
Maël Le Ménédeu (2022-25) // Charline Sire (2021-25) // Cédric Ollier (2021-24)
Marie Le Roy (2019-22) // Julie Pineau (2019-22) // Axia Marlin (2019-22)
Gwladys Nizou (2018-21) // Evan Lelong (2017-20) // Jonathan Mendy (2016-19)
Laura Grenier (2016-19) // Thomas Le Bihan (2015-18) // Amaury Guillou (2015-18)
Mariane Le Fur (2014-17) // Mathieu Frindel (2012-15) // Nathalie Camus (2011-14)
Mélissa Roger (2010-13) // Jacky Pouessel (2008-11)
PostDocs (since 2010) :
Federica Battistin (2023-25) // Dyhia Amrane (2023-25)
Julie Pineau (2022-25) // Maher Hojorat (2022-24)
Nancy Al Haddad (2023-24) // Nadège Hamon (2020-22)
Richard Knighton (2018-19) // Alexandra Vuillamy (2018-19)
Thibault Troadec (2016-17) // Fatima Oukhatar (2013-14)
Mustapha Allali (2013) // Zakaria Halime (2012-13)
Ronan Marion (2012-13) // Luis Lima (2010-11)
Contacts
-
Raphaël TRIPIER
Professeur - Vice-président recherche et innovation - Responsable Groupe thématique "Macrocycles Azoté et Coordination"
raphael.tripier@univ-brest.fr








